Introduction
Reproductive health is a major concern in male patients with sickle cell disease (SCD). SCD is associated with pubertal delay (Soliman 2021), however, limited large-scale studies exist on the age of puberty onset in male SCD patients. Additionally, men without hydroxyurea (HU) therapy often experience decreased fertility, with less than 10% having normal semen analysis results according to WHO guidelines (Berthaut 2008). HU treatment may further reduce fertility in many patients (Berthaut 2017).
To address these concerns, various fertility preservation strategies have been developed for male SCD patients on HU, including sperm cryopreservation for pubescent boys and adults before starting HU, and temporary HU discontinuation when therapy is initiated in infancy for sperm banking, at best with monthly transfusion during the period without HU.
Methods
The ongoing European Sickle Cell Disease Cohort - Hydroxyurea Extension (ESCORT-HU Extension) study is a multicentric, prospective, non-interventional study aiming to better document the safety profile of HU. It focuses on poorly understood or documented risks, including puberty and fertility. Around 2000 HU-treated patients are expected to participate over a 5-year duration.
The study includes both patients who were previously enrolled in the initial ESCORT-HU study and new patients who meet specific inclusion criteria.
Results
As of June 28, 2023, a total of 1860 patients were included in the study in France (92% of patients), Germany, Greece and Italy.
Median age of patients at inclusion was 24 [IQR: 14-40] years with 44.4% being males, and 87% having the HbSS genotype.
At enrollment, 455 (56.5%) males were in the pubescent stage, and 72 males transitioned into the pubescent stage since then.
Median duration of HU exposure at the time of analysis was 8.5 [IQR: 6.4-11.6] years.
Onset of puberty
The mean age at Tanner V stage did not significantly differ between males who reached puberty before starting HU and those who reached puberty after (15.0 [IQR: 14.0-16.0] years vs. 15.2 [IQR: 14.0-16.0] years, respectively).
Semen cryopreservation
Among the 527 pubescent males, 293 started HU at or after 17 years old with 117/240 (49%) who had a cryopreservation. 234 started HU before the age of 17 years, 23/207 (11%) had a cryopreservation. Unknown data were reported for 80 patients. Median age at cryopreservation was 26.0 [IQR: 21.0-33.0] years.
Semen analysis
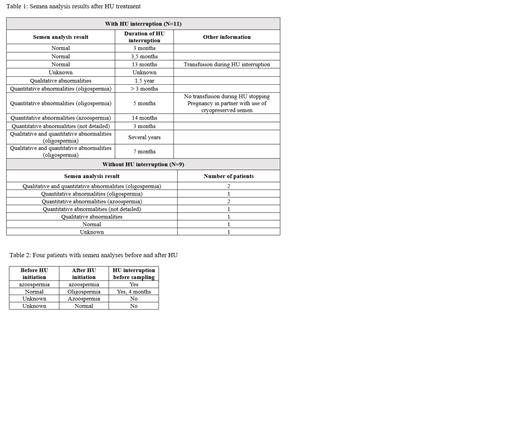
A total of 104 semen analysis were reported in the database with 66 performed before HU treatment initiation and 24 after, with 9 of them without any HU interruption prior the sampling. In 14 cases, the documentation on HU stopping was not available.
Among the 66 patients tested before HU treatment initiation, results were not available in 34. They were normal in 25 (78%), abnormal in 7 (22%) (4 qualitative anomalies, 2 azoospermia, 1 both quantitative and qualitative anomalies).
For the 24 patients who performed semen analysis after HU initiation, 11 temporarily discontinued the treatment for the procedure, with 3 having normal results, 7 having abnormal results, results not available in 1. One of the patients with oligospermia used successfully its previously cryopreserved semen for his partner's pregnancy. Nine patients did not stop HU before the procedure, with 7 having abnormal results, 1 normal, and result not available in 1. Information on HU interruption during the cryopreservation was not available for 4 patients (Table 1).
Four patients have semen analysis results before and after HU treatment (Table 2).
Conclusion:
This study shows no impact of HU on the age of puberty in males. Data on fertility need to be completed but we show that semen cryopreservation has been well adopted in Europe, since approximately 50% of patients over the age of 17 at HU initiation included in France have undergone this procedure, which is free in France.
We confirm a significant proportion of semen abnormalities but not in all cases. The impact of HU on fertility is complex, and cryopreservation appears to be beneficial in mitigating potential adverse effects, with at least one baby in our cohort born after use of cryopreserved semen.
Disclosures
De Montalembert:Novartis: Consultancy; Vertex: Other: Steering committee; Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bartolucci:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird: Consultancy; Roche: Consultancy; Emmaus: Consultancy; GBT: Consultancy; Jazz Pharma: Consultancy; INNOVHEM: Current equity holder in private company; Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees. De Luna:GBT: Consultancy; VERTEX: Consultancy; Pfizer: Other: Principal Investigator HEMOPROVE trial. Habibi:GBT: Consultancy; Add Medica: Honoraria; novartis: Consultancy. Etienne-Julan:Addmedica: Membership on an entity's Board of Directors or advisory committees. Cannas:Addmedica: Membership on an entity's Board of Directors or advisory committees. Arlet:Addmedica: Research Funding; Novartis: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Vertex: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal